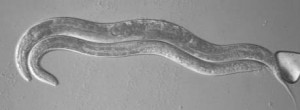
The process of sexual reproduction involves a host of requirements that must be met before an oocyte and sperm can fuse together to form a zygote capable of further functional development. One such requirement that remains incompletely understood, despite years of study in multiple model organisms, is the complex cellular program of meiosis. Meiosis is the specialized cell cycle by which the haploid gametes (oocytes and sperm) are produced. It is not only of crucial importance to successful sexual reproduction or propagation of a species, but also to human health. Defects during the meiotic divisions have serious deleterious outcomes such as infertility, spontaneous miscarriages, birth defects (such as Down Syndrome), and even tumorigenesis. In addition, many of the mechanisms that regulate meiosis are reutilized in the mitotic cell cycle, and improper regulation of mitosis is a critical factor in many diseases, such as cancer. Furthermore, comparisons of the general strategy and currently known factors involved in meiosis shows a high degree of evolutionary conservation between mammals and more amenable model organisms such as Drosophila and Caenorhabditis elegans.
We utilize the nematode C. elegans and are taking an interdisciplinary approach involving genetics, molecular biology, cytological, and biochemical techniques to study aspects of reproduction, including oocyte meiotic arrest, oocyte maturation, RNA-binding protein function, physiological germline apoptosis and the role of the proteasome.
There are three main active areas of investigation in the laboratory:
- Can we further elucidate the function of the meiotic inhibitory kinase WEE-1.3 during reproduction? Current questions include: why does precocious oocyte maturation result in fertilization incompetent oocytes? What additional regulators or interacts of WEE-1.3 exist in the oocyte maturation pathway? We have recently generated endogenously WEE-1.3 GFP tagged strains to help us further study the role/function of WEE-1.3.
- What is the role of the RNA-binding protein ETR-1 in reproduction? We have identified a novel role for ETR-1 in hermaphrodite reproduction and in physiological germline apoptosis (Boateng et al., 2017). Animals depleted of ETR-1 show reduced fertility and increased numbers of apoptotic germ cell corpses. Currently we are trying to elucidate the isoform specific roles of this complex protein (19 splice isoforms) by using CRISPR/Cas9 to endogenously GFP tag different isoforms, investigating the role of this protein in male spermatogenesis and spermiogenesis, and beginning to study the function of this gene in apoptosis.
- Uncovering non-proteolytic roles for subunits of the 26S proteasome during reproduction. We previously identified that certain subunits of the 19S lid of the proteasome seem to be playing a role in reproduction (Allen et al., 2014). We are now trying to further elucidate what these lid subunits are doing to ensure a reproductively capable animal. Additional studies have identified a role for the 19S subunit RPN-12 in hermaphrodite sex determination (manuscript in preparation).
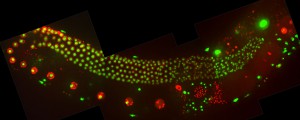
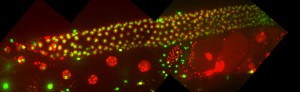
Images: Dissected germlines from control depleted animals (top) or WEE-1.3-depleted animals (bottom) that have been stained with markers for precocious oocyte maturation (phospho-histone H3 in red and the nucleolus in green).
C. elegans is an ideal system to study oocyte maturation, fertilization and apoptosis as many aspects of gametogenesis and embryogenesis can be observed directly in a living animal. As function is conserved in the currently identified meiotic pathways across the animal kingdom, C. elegans is ideal for identifying novel components of oocyte meiotic maturation and cell cycle regulation. The information gained from these studies will not only contribute to our understanding of the events of meiosis and fertilization, but will strongly benefit multiple fields- the reproductive medicine, the cell cycle, and the cancer fields.
Dr. Allen is also interested in educational research, and actively incorporates CUREs (course-based undergraduate research experiences) into her courses at Howard University. This interest in active learning techniques has led her to join the Genomics Education Partnership (GEP) where she is involved in introducing undergraduates at Howard to genomics research through a CURE laboratory in her BIOL200 Genetics course. She also has an active role in the GEP board, serving on the Assessment Committee and as a liaison for ensuring all participating institutions have proper IRBs in place to conduct the research.